
MSK molecular geneticist Debyani Chakravarty, PhD, and colleagues analyzed the clinical actionability of more than 47,000 solid tumors.
What proportion of cancer patients are eligible to benefit from precision oncology? There is an ongoing debate on this issue, partly due to a lack of consensus on which gene mutations are clinically actionable.
To answer the question, researchers at Memorial Sloan Kettering Cancer Center (MSK) recently analyzed the clinical actionability of more than 47,000 solid tumors sequenced with the MSK-IMPACT® (which stands for Integrated Mutation Profiling of Actionable Cancer Targets) clinical assay using the 2017 and 2022 versions of MSK’s precision oncology knowledge base OncoKB™. (1) Their results were recently in Cancer Discovery. (2)
The analysis revealed the fraction of clinically actionable tumors, defined as harboring an OncoKB™ Level 1 or Level 2 predictive biomarker of therapy response, increased significantly, from 8.9% to 31.6%, between 2017 and 2022. (2)
Refer to the figure below to learn about OncoKB™ Therapeutic Levels of Evidence:
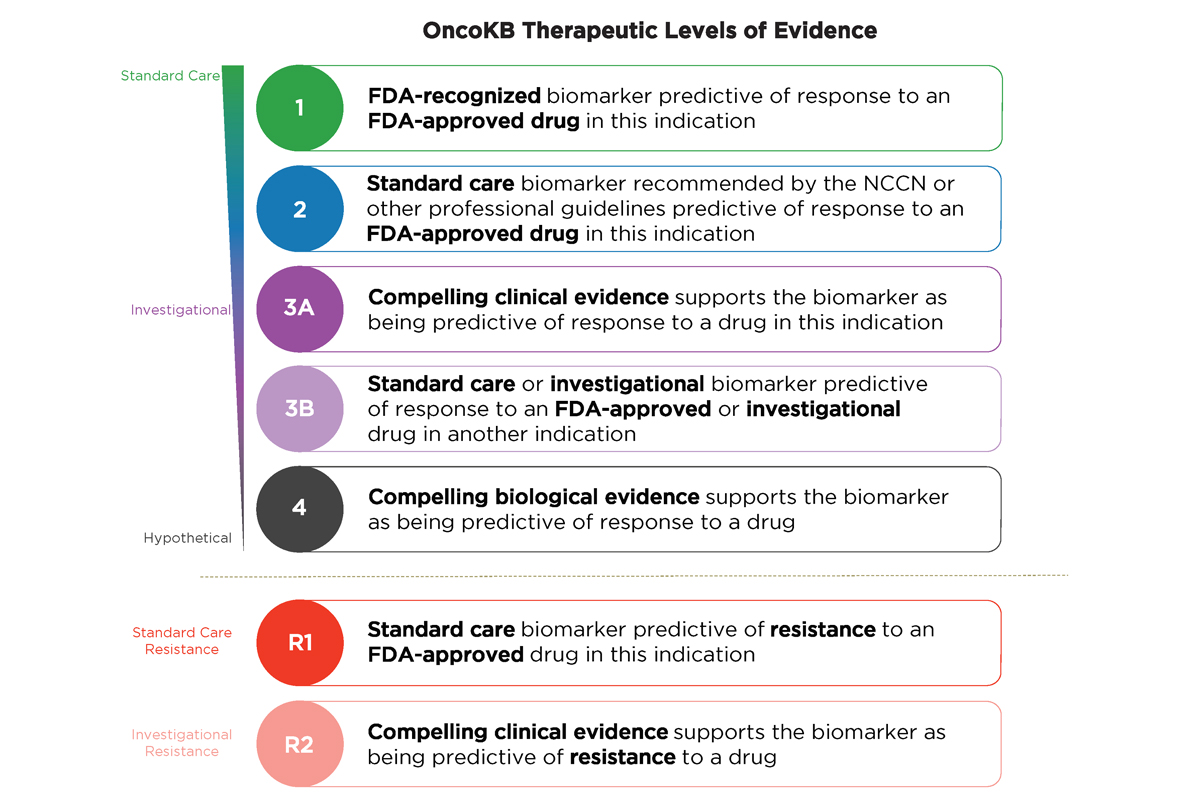
“This impressive rise in actionable mutations over the most recent five-year period is a testament to the advent of large-scale molecular profiling studies, advances in clinical trial design, such as basket trials across tumor types, and the lowered costs coupled with progressive improvement in the depth of coverage and accuracy of detection of next-generation sequencing-based diagnostic assays ,” said MSK molecular geneticist Debyani Chakravarty, PhD, senior author of the paper. “Our findings also underscore the continuing need for new precision therapeutics, especially for cancers with currently undruggable driver mutations.”
Study Design
First, Dr. Chakravarty and colleagues reviewed all oncology drugs approved by the U.S. Food and Drug Administration (FDA), starting with trastuzumab in 1998 through 2022. They assessed drug labels, consensus guidelines, and the scientific literature to identify the subset of drugs that require biomarker testing for patient selection and termed these “precision oncology therapies”. The authors further classified precision oncology therapies based on whether the drug targets a gene mutation previously considered undruggable or employed a novel mechanism of action. (2)
Next, the MSK investigators quantified the proportion of drugs considered clinically actionable using the March 2017 and October 2022 versions of OncoKB™ to annotate all mutations, focal copy number alterations, and structural variants in 47,271 tumor samples sequenced with the MSK-IMPACT® platform and available publicly as part of AACR Project GENIE® (Genomics Evidence Neoplasia Information Exchange). (2)
Note that targeted therapies that do not require molecular testing before treatment, such as chimeric antigen receptor (CAR) T cell therapies and some antibody-drug conjugates, were not included in this analysis. (2)
Study Results – Increasing Pace of Precision Oncology Drug Approvals
Among 198 new oncology drugs approved, 164 were classified as targeted therapies based on their ability to inhibit or bind to a specific protein. Within that 164, 86 (52%) were further identified as precision oncology drugs, of which 69 (80%) had a genomic biomarker detectable by next-generation sequencing. These 69 therapies were most effective in tumors with alterations in 45 distinct genes or with microsatellite-high or tumor mutation burden-high genomic signatures. (2)
The MSK investigators classified these 69 drugs as follows: 33 (48%) as first-in-class, 7 (10%) as having a distinct mechanism of action targeting previously actionable alterations, and 29 (42%) as follow-on/resistance precision therapies. (2)
Their analysis revealed precision oncology drug approvals increased significantly since 1998, especially during 2017 to 2022: (2)
- 1998 to 2012: While the FDA approved one or two first-in-class or follow-on drugs annually, if any, this period was notable for defining the concept of precision oncology with the approval of watershed drugs including imatinib, vemurafenib, and erlotinib.
- 2012 to 2017: Approvals were mainly for drugs targeting alterations already considered actionable based on prior approvals, except for novel PARP inhibitors for BRCA1/2-mutant ovarian cancer, which had a novel mechanism of action.
- 2017 to 2022: The pace of approvals increased rapidly, rising to a median of 8 annually. In total, the FDA approved 23 first-in-class drugs targeting 31 novel biomarker-selected patient populations and 18 follow-on drugs. In 2020 alone, the FDA approved 12 drugs — 8 first-in-class drugs and 4 follow-on drugs.
Study Results – Significant Expansion in Clinical Actionability
The proportion of clinically actionable tumors rose significantly during 2017 to 2022: (2)
- The fraction of tumors with a somatic DNA mutation, fusion, or copy number alteration considered eligible for a Level 1 or Level 2 standard care targeted therapy or immunotherapy or a clinical trial with promising clinical data (Level 3A) almost doubled from 18.1% to 35.9%.
- There was an almost four-fold increase in the proportion of tumor samples with an FDA-recognized Level 1 biomarker of drug response, from 7.7% to 30.2%. The most significant contributor to this increase was the approval of pembrolizumab for tumor mutation burden-high tumors.
- The proportion of tumors with non-actionable drivers declined by about half, from 44.2% to 22.8%.
Notable first-in-class drug approvals that drove the significant increase in clinical actionability during 2017 to 2022 included the following: (2)
- Alpelisib for PIK3CA-mutant breast cancer (28% of breast cancers),
- Erdafitinib for FGFR3-mutant bladder cancer (24% of bladder cancers),
- Pemigatinib, infigratinib, and futibatinib for FGFR2-fusion-positive cancers (6%),
- Ivosidenib for IDH1-mutant cholangiocarcinoma (10% of cholangiocarcinomas), and
- Selpercatinib and pralsetinib for RET-fusion-positive lung and thyroid cancers (2% and 11%, of those cancers, respectively).
- Sotorasib, the first KRAS G12C-targeted agent, increased the fraction of non-small cell lung cancer samples with a Level 1 biomarker predictive of treatment response by 12 percentage points.
- Approvals for agnostic tumor types rose, providing more patients with rare or unknown primary tumors with new treatment options. For example, among 1,423 patients with cancers of unknown primary, 305 (21.4%) became eligible for the first time to receive an FDA-approved Level 1 precision oncology drug.
More Work Ahead
Dr. Chakravarty and colleagues also examined the percentage of non-actionable driver genes in samples with limited clinical actionability, defined as Level 3B or Level 4 OncoKB™ Therapeutic Levels of Evidence. They found the most frequently mutated genes were TP53 (43.2%), KRAS (19.2%, and CDKN2A (12.2%). (2)
“While the increase in clinical actionability of precision oncology drugs during 2017 to 2022 is impressive, more progress will depend on the development of new therapies driven by alterations that do not respond to small molecule inhibitors that work well for gain-of-function kinase inhibitors,” Dr. Chakravarty said. “Further, one-third of tumors in our study had more than one clinically actionable mutation, indicating that more selective and less toxic combination strategies will be required for future gains.”
MSK continues be at the leading edge of the precision oncology field. For example, thoracic oncologist and early drug development specialist Alexander Drilon, MD, led a novel trial testing the experimental SHP2 inhibitor PF-07284892, which was designed to overcome bypass-signaling-mediated resistance when combined with targeted therapies. Importantly, he designed the trial to allow early introduction of combination therapy: patients whose cancer progressed on SHP2 monotherapy during the lead-in period were permitted to receive oncogene-directed targeted therapies that had previously failed. This first-in-human trial (NCT04800822) included patients with ALK fusion-positive lung cancer, BRAF V600E-mutant colorectal cancer, KRAS G12D-mutant ovarian cancer, and ROS1 fusion-positive pancreatic cancer who previously developed targeted therapy resistance. The combination approach resulted in rapid tumor and circulating tumor DNA responses and extended the duration of clinical benefit. The results were published recently in Cancer Discovery. (3)
MSK at the Forefront of Precision Oncology
The study was a collaboration between 26 authors from MSK’s Marie-Josée and Henry R. Kravis Center for Molecular Oncology, Department of Pathology and Laboratory Medicine, Department of Medicine, Department of Epidemiology and Biostatistics, and Human Oncology and Pathogenesis Program.
MSK-IMPACT® is the first laboratory-developed tumor profiling test to receive FDA approval. Its customized hybrid capture method provides room for growth as new targets are discovered by the Cancer Genome Atlas and the Cancer Genome Consortium.
In October 2021, OncoKB™, MSK’s public human genetic variant database, became the first somatic human variant database to be recognized by the FDA.
AACR PROJECT GENIE® is a publicly accessible cancer registry of genomic data and anonymized clinical records. Initially a collaboration among eight leading international cancer centers, including MSK, there are now 19 contributing research institutions.
The study was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748, OncoKB™ commercial licensing fees, a Prostate Cancer Foundation Challenge Award, and the MSK Marie-Josée and Henry R. Kravis Center for Molecular Oncology. Dr. Chakravarty reported a consulting or advisory role for Mendendi Medical Travel. Refer to the paper for disclosures from other authors.